TL; DR
Most atoms never change, some do. The ones that change do at a rate you can measure, this allows you to tell how old the stuff is that they belong to. You can use this method to tell how old the earth is and how old things like human remains are too. The world is amazing.
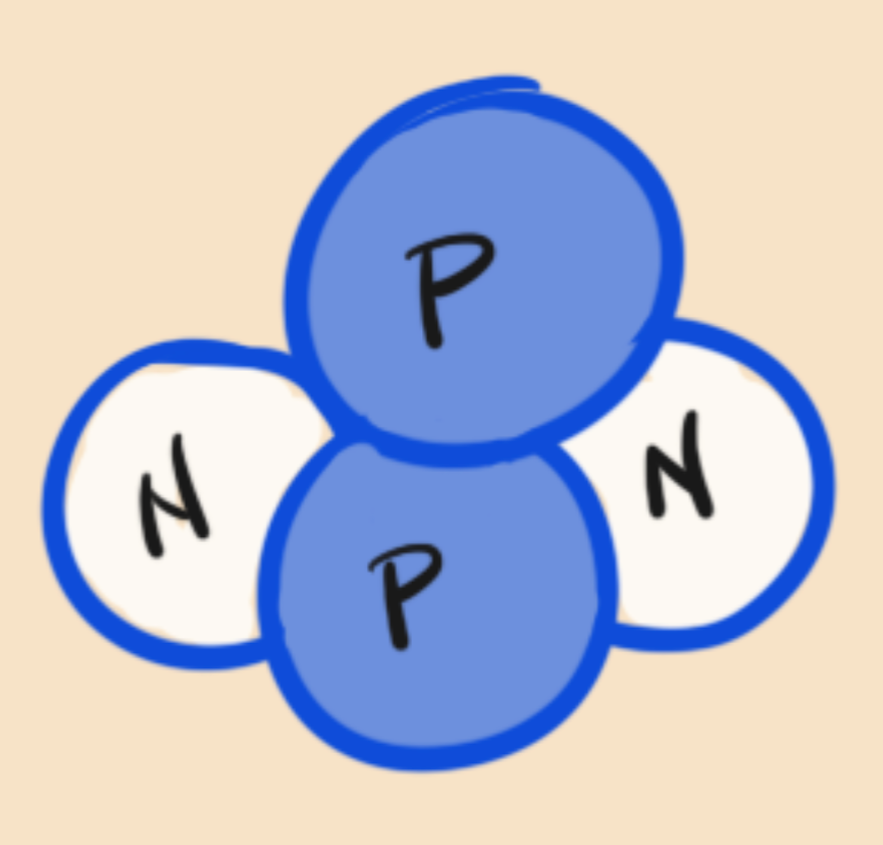
Think of an atom like this. It has protons (P) and neutrons (N). Remember from here how protons repel other protons? Yep, there is another force that we call the nuclear force. It is so strong that it makes those protons stick together.
In fact it is so strong, that most of the atoms from the beginning of the universe are still exactly the same as they have been since the start.
Measuring Really Old Stuff
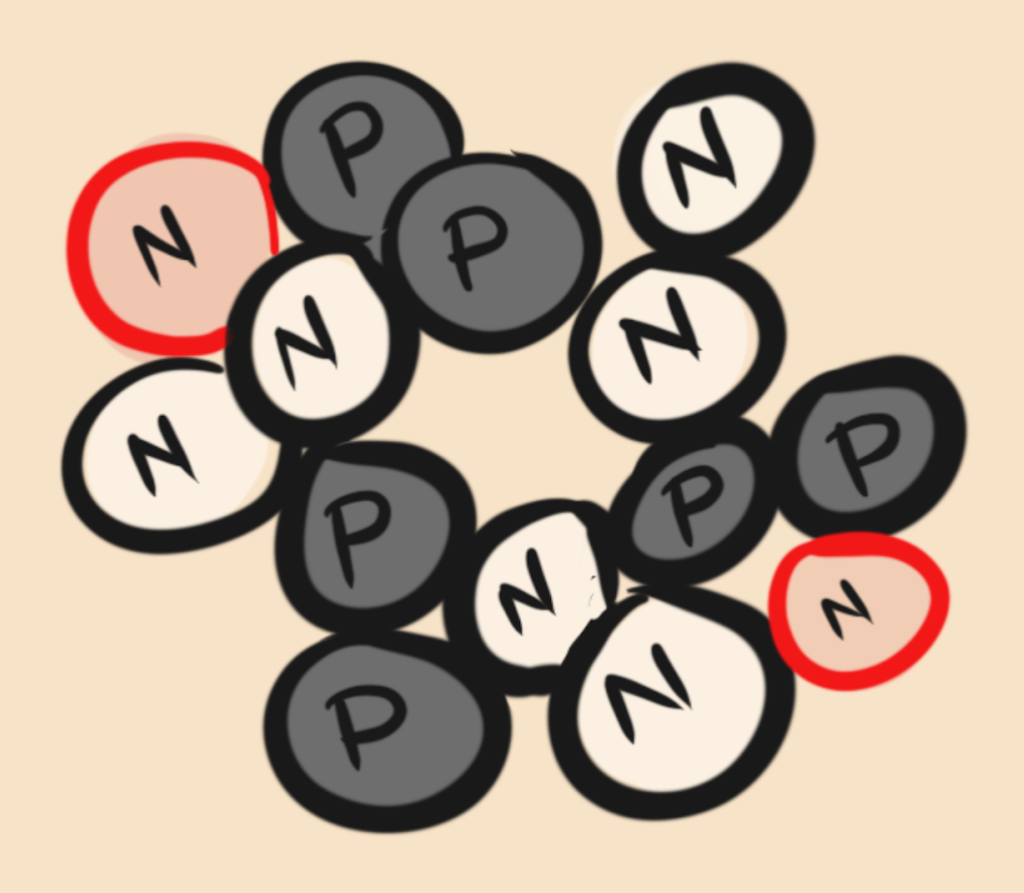
There are some substances that do change over time. An example is Uranium (atomic #92 for it’s 92 protons) that will lose protons and become another element entirely, it keeps losing protons until it becomes Lead (Atomic #82 for it’s now 82 protons). An atom that changes like this is called radioactive.
It takes a long time though for a chunk of uranium to change. It is made of a gigantic number of atoms and you can’t tell when any one atom is going to change. Because there are so many of them you know on average how often at least some of them will change. It happens so predictably that you can measure time with it. Like this: say you find a hunk or uranium in your backyard and before dying from it (yeah pretty dangerous stuff), you measure that it has 100 radioactive atoms in it. For any 50 of them to decay it will take, get this, 4.5 billion years. In other words, 4.5 billion years is how long it takes half of the atoms in your chunk of backyard-death-rock to lose some of its protons and become a new element. That is called a half life. We can use these methods to determine the age of really old things like the earth.
Measuring Sorta Really Old Stuff
Other atoms change over time also. An especially useful one to measure is carbon. Interestingly enough, you are 18% carbon. Plants & animals have lots of carbon too. it looks like this:
Because it has 6 protons & 6 neutrons it is called carbon-12
For every trillion or so normal carbon atoms there is a carbon atom that that has two extra neutrons. (6+8=14) so it is called carbon-14, carbon-12 doesn’t change/decay but this 14 fella does. Unlike uranium though carbon-14 just turns back into carbon-12.
Of all the carbon in trees and animals and you – most of it is carbon-12.
Where do these carbon-14’s come from?
Cosmic rays cause a chain reaction that regularly creates new carbon 14’s in our atmosphere. Since trees eat carbon-dioxide they eat a bunch of these carbon 14’s too. Animals and humans fill up our stock of them when we eat plants. When we die, we stop eating carbon 14’s and that is where the time measuring magic starts.
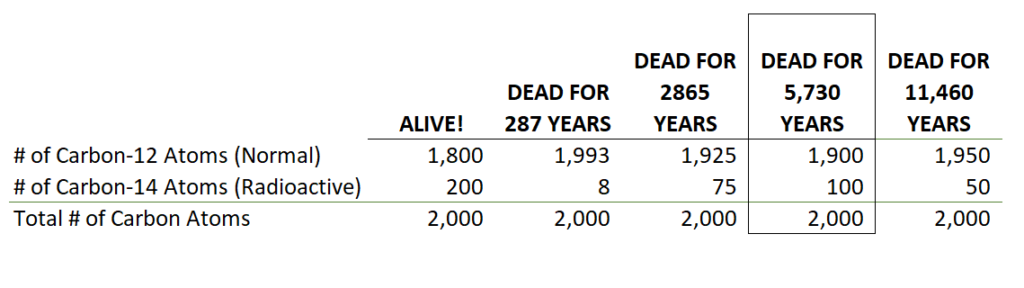
The hourglass stays topped off as long as you keep eating from the plants that eat carbon dioxide. As the 14’s decay you can compare a sample dead thing to a sample living thing and see how old the dead thing is. This table is purposely simplified (90% carbon-12 and 10% carbon-14) to give you the general idea1In reality about 15 of these atoms decay every minute for every gram of carbon:
A sample of carbon loses it’s carbon 14’s and gets to a half-life after 5,730 years. When it loses another half life (like in the table) you can add on another 5,730 years and add it up to get: 11,460 years.
Humans have used this method to date things from human remains to trees to even old ships.
So what?
Honestly, I just love how crazy interesting it is that Nature left so many clues that we can unwind. It is unbelievable that there are giant swaths of time that we know nothing about people who were genetically very much like us before there was any written history. There are some spectacular stories that are completely lost to history. Only the traces of bones and archaeology give us slight hints. Wow!